Flow Cytometry
What is an antibody?
Last update: October 1st, 2019
The distributed nature of the hematopoietic system allows its analysis by flow cytometry. Many surface proteins and glycoproteins on erythrocytes, leukocytes, and platelets have been studied in great detail due to the availability of monoclonal antibodies directed against these surface proteins. Antibodies against intracellular proteins are also commercially available and permit analysis of an increasing number of intracellular markers.
1. Antibodies:
Antibodies allow scientists to detect a specific antigen, making them useful for characterizing the proteins on the surface of live cells. The technology involves creating an antibody reagent that will bind to a specific structure (antigen) known to be present on the kind of cell of study. This technology is the key to developing biological reagents that could be used not only to identify cells, but also to analyze the function of that cell.
A primary antibody is an immunoglobulin that specifically binds to a particular protein or other biomolecule of research interest for the purpose of purifying or detecting and measuring it.
Polyclonal vs. Monoclonal for Flow Cytometry
Monoclonal antibodies (mAb or moAb) are antibodies that are made by identical immune cells that are all clones of of one hybridoma cell of origin. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies are comprised of a mixture of antibodies that bind to different epitopes of the same antigen and are usually made by several different plasma cell (antibody secreting immune cell) lineages.
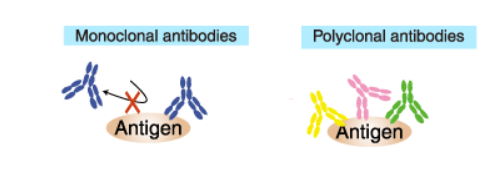
These facts make polyclonal antibodies great for quick and cost-effective detection of cell surface markers. However, an advantage of monoclonal antibodies (mAbs) compared to polyclonal antibodies is that they provide cleaner data as they produce less non-specific background noise. In order to do complex experiments detecting multiple antigens, or just to get a primary antibody that is compatible with certain fluorescently labeled secondary antibodies, the proper host species for each antibody must be considered.
Commercially available primary antibodies are produced in many ways, and these differences yield antibodies having unique characteristics that affect their suitability for different applications. Knowing the characteristics of each type of antibody is important for choosing the best one for your research.
Recombinant antibodies provide a high specificity, high sensitivity option all while being animal origin-free (AOF). They are AOF because all recombinant antibodies are derived from DNA. This is beneficial because there are fewer animal immunizations needed leading to less lot-to-lot variability. Instead, each new lot of recombinant antibody comes straight from the same genetic source and has the same bioactivity, specificity, and quality as the previous lots.
2. Fluorochromes:
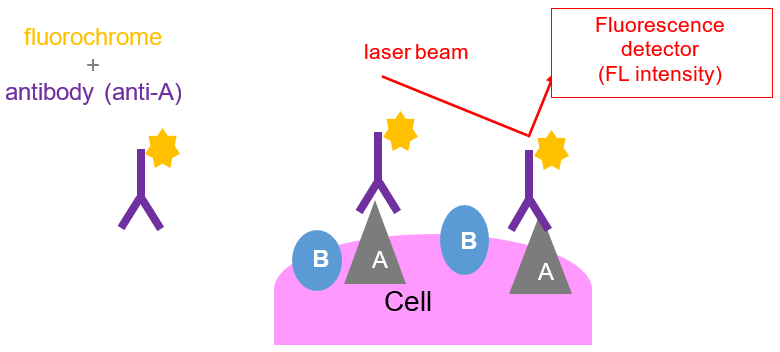
In order to track the cells that have bound to the antibodies, they are directly linked to a fluorescent dye (fluorochrome) or they can be visualized by binding to a secondary antibody that is labeled with a fluorescent marker. Fluorochromes emit fluorescent light when excited by a laser and will be picked up when that cell passes through the laser beam of the flow cytometer.
Fluorochromes differ from each other with respect to the color of light that they will emit. For example, OC515 (Cytogos fluorochrome), will emit green light, whereas APC-C750 (another Cytognos fluorochrome), will emit red light when it is exposed to the red or violet laser respectively. The use of different fluorochromes helps researchers distinguish between cell populations or different cellular activities.
Fluorochromes can be classified according to their mechanism of action: those whose fluorescence increases with binding to specific cell compounds such as proteins (fluorescein isothiocyanate [FITC]), nucleic acids (propidium iodide [PI]), and lipids (Nile Red); those whose fluorescence depends on cellular physiological parameters (pH, membrane potential, etc.); and those whose fluorescence depends on enzymatic activity (fluorogenic substrates) such as esterases, peroxidases, and peptidases. Fluorochromes can also be conjugated to antibodies or nucleotide probes to directly detect microbial antigens or DNA and RNA sequences.
A fluorochrome:
- Absorbs energy from laser.
- As a result, it excites and emits the absorbed energy at a longer wavelength.
- Finally, this light is collected by the detector, which registries the fluorescence intensity.
- Emission and absorption spectra are fluorochrome-specific: cells can be tagged with different antibody-fluorochrome combination to detect different proteins on the cells
- The more occupied positions, the more intensity signal is obtained.
- Surface and intracellular staining: not only proteins from the membrane surface can be tagged, also from the cytoplasm (permeabilization).
Resources
Publications:
- Adan A, et al. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017 Mar;37(2):163-176. Go to publication
- Cossarizza A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol. 2017 Oct;47(10):1584-1797. Go to publication
- Brown M and Wittwer C. Flow Cytometry: Principles and Clinical Applications in Hematology. Clinical Chemistry. 2000 April;46:8(B)1221–1229. Go to publication
- Howard M. Shapiro Practical flow cytometry, 4th ed. John Wiley hi Sons, Inc. 2003 August; 736 Pages. Go to publication

