In Vitro Diagnostic Medical Devices Regulation
EU 2017/746
In May 2017, the European Union published the In Vitro Diagnostic Medical Devices Regulation EU 2017/746 (IVDR) which repeals the In Vitro Diagnostic Medical Devices Directive 98/79/EC (IVDD) to establish a more robust, transparent, predictable and suitable regulatory framework for IVD Medical Devices.
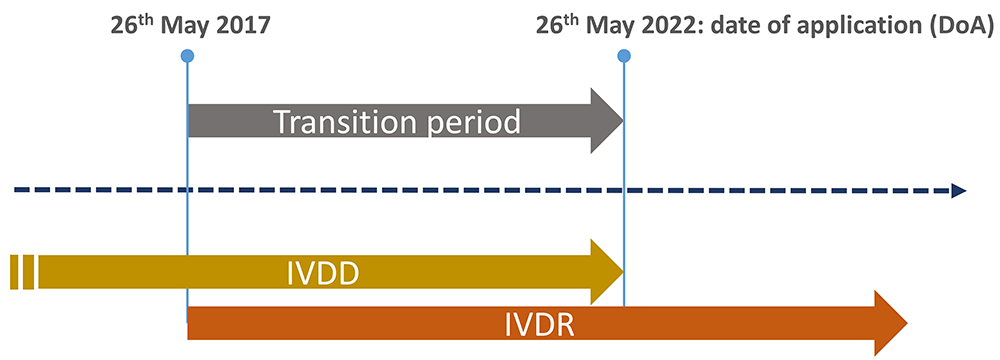
The unprecedented magnitude of the challenges linked with the situation imposed by the COVID-19 pandemic, the limited capacity of notified bodies and the overall lack of sufficient readiness of the regulatory system to effectively implement the EU IVDR, triggered the approval of the Regulation (EU) 2022/112 of 25 January 2022 which amended the EU IVDR.
This amendment extends the transitional provisions to allow most IVDs (class A sterile, B, C and D) with their EC Declaration of Conformity under Directive (EC) 98/49 (IVDD) to be placed on the market and put into service for additional time depending on their risk class under the IVDR. IVD products can benefit from these transitional provisions provided that, they comply with the IVDD and there are no significant changes in the design and intended purpose.
- IVDD class B and A sterile devices– may be placed on the market or put into service until 26 May 2027. IVDs which are already placed on the market before that date may continue to be made available or put into service until 26 May 2028 (sell-off period).
- IVDD class C devices– may be placed on the market or put into service until 26 May 2026. IVDs which are already placed on the market before that date may continue to be made available or put into service until 26 May 2027 (sell-off period).
- IVDD class D devices or devices that have IVDD certification– may be placed on the market or put into service until 26 May 2025. IVDs which are already placed on the market before that date may continue to be made available or put into service until 26 May 2026 (sell-off period).
Cytognos and the new IVD Regulation
Cytognos is working hard to continue offering our diagnostic kits, antibodies and Infinicyt™ under the IVDR, while we benefit from the amended transitional period for certain products.
All products in Cytognos CE-IVD Product List bear a CE-IVD mark and they are in compliance with the EU IVDD (the ones that qualify for the transitional period) or EU IVDR (class A devices).

